The burning of polymers could be described simply as a kind of rapid, exothermic, oxygen induced degradation process. Somewhat like heat-induced processing oxidation and ultraviolet degradation, combustion is a free radical sustained process: (Somewhat like heat-induced processing oxidation and ultraviolet degradation, combustion is a free-radical sustained process:
- Fire is initiated by high temperatures (typically .320°C, for polyolefins), which decompose the polymer into gaseous species that are combustible.
- Hydrogen radicals (H•) are pulled by heat from these decomposition products and combine with oxygen molecules.
- This reaction creates further radicals (oxygen and hydroxy, O• and HO•), which react with carbon monoxide exothermically.
- This reaction creates heat and more H• radicals, propagating a chain reaction that causes additional burning, unless the combustion reaction is interrupted or interfered with by flame-retarding additives.
Types of flame retardant additives
Flame retardant additives are divided into halogen and non-halogen bases. A brief explanation of this issue can be found below:
Halogen-Based FRs
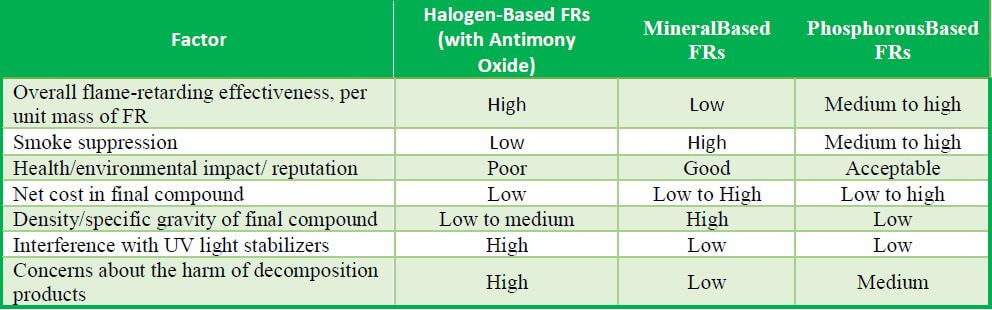
Like heat and light stabilizers, FRs containing bromine or chlorine act as free-radical scavengers. They interrupt combustion essentially by replacing free hydrogen and hydroxyl radicals with halide (bromide or chloride) radicals that prevent the combustion reaction from continuing. This reaction cycle makes H-FRs highly effective for extinguishing or suppressing combustion. In particular, brominated compounds are favored because they dissociate more easily. However, the acidic products of H-FRs can interfere with the activity of basic hindered amine light stabilizers (HALS) in the formulation, and often these FRs bloom to the surface of the polymer.
Inorganic-Based FRs
Inorganic compound synergists are typically added to an FR package to increase the halogens’ radical scavenging effectiveness. Specifically, antimony trioxide (Sb2O3) reacts with hydrogen halides to form new radical scavengers that assist in suppressing combustion. For example, in bromine-based FR formulations (in which flame-suppressing hydrogen bromide (HBr) is produced), antimony trioxide reacts with HBr, ultimately creating antimony halides and neutralizing free radicals through a series of reactions. Other additives added along with antimony trioxide reportedly can increase Sb2O3’s synergistic effect or can replace it as lower cost, Sb-free alternatives; these include ferric oxide, zinc borate, and barium metaborate. And because standard-sized Sb2O3 particles tend to make plastic articles opaque, colloidal-size antimony oxides have been proposed for creating translucent H-FR plastics. Sb2O3 with particle sizes of 0.8-1.0 microns behaves as white pigments. But a variant form, antimony pentoxide (Sb2O5), with particle sizes under 0.15 microns, can be used to produce translucent FR-P.
Phosphorous-Based FRs
Phosphorous-based FR systems based on phosphate salts can be useful in FR polyolefins. P-FRs decompose in heat to produce an expanding, intumescent foam char on the burning plastic’s surface, which isolates the underlying plastic from further combustion. Since the burning of PP is more of a surface oxidation process than that of PE, intumescent FRs are more suitable for PP than PE. These FR systems can be as effective as halogenated FR systems and thus are their major competitors. They suppress smoke better and do not have the corrosive byproduct and UV-stabilizer interference issues of H-FRs. Their flexibility has caused their formulators to focus on reducing their typical limitations, such as low decomposition temperature, high loading rates, and water extraction. Generally, these FRs become active as the polymer starts to decompose from heat, leading to reactions which form a foamed charred surface. By its thickness and insulating quality, the char blocks the release of smoke, heat, burning gases, and other off-gases. This intumescence is especially useful in electronics applications where nearby parts could be susceptible to burning or damage. Also, P-FRs do not interfere with HALS light stabilizers as halogenated FRs do
Comparison of Fundamental FR Types for Polyolefins
Property Effects and Co-additive Interaction
Because FRs are not added in order to be part of a compoundstrengthening system, and because they must often be used at high loadings, it is not surprising that they have unwanted effects on most mechanical properties. Even though FR additives may be of various types from various suppliers, certain general physical property changes commonly result from the use of FRs:
- High loadings of FRs can increase flexural modulus and rigidity (especially mineral FRs).
- High loadings of FRs can reduce strength.
- Low and high loadings of FRs can reduce impact resistance, sometimes severely
For instance; Mechanical Property Comparison of PP Containing Various FRs:
| Material | Flexural Modulus (GPa) | Tensile Strength (MPa) | Notched Izod Impact (J/m) |
| PP (homopolymer, No FR) | 1.2-1.4 | 33 | 44-48 |
| PP+ 10% H-FR (with 3% sb2O3) | 1.2 | 33 | 32 |
| PP+ 20% P-FR | 1.9 | 30 | 37 |
| PP+ 30% P-FR | 1.9 | 30 | 27 |
| PP150% MDH | n/a | n/a | 38 |
| PP160% MDH | 3.7 | 22 | n/a |
| PP145% MDH115% talc | 4.6 | 23 | n/a |
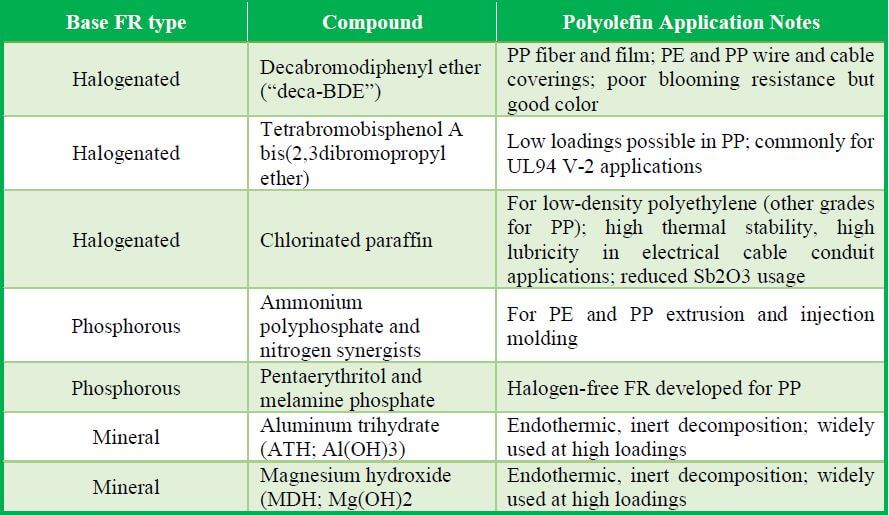
Common FRs Most Relevant for Polyolefins
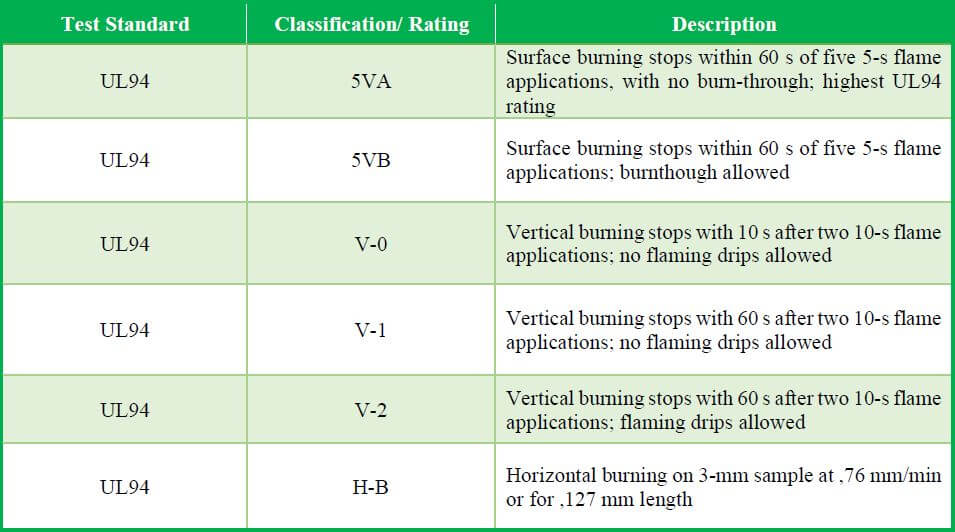
Flammability Tests Relevant to Polyolefin Applications
Buy flame retardant masterbatch
Flame retardant masterbatch is produced in several grades by respected manufacturers, so if you consult with our expert experts and know exactly what you need, you can buy flame retardant masterbatch. Additionally, technical information is provided with this product. Our experts can answer your questions about the flame retardant masterbatch price and purchase.
Dan Polymer company’s flame retardant masterbatches
|
Description |
Product |
|
flame retardant masterbatche based on ABS |
Dan-ABS-FR |
|
flame retardant masterbatche based on PP |
Dan-PP-FR |